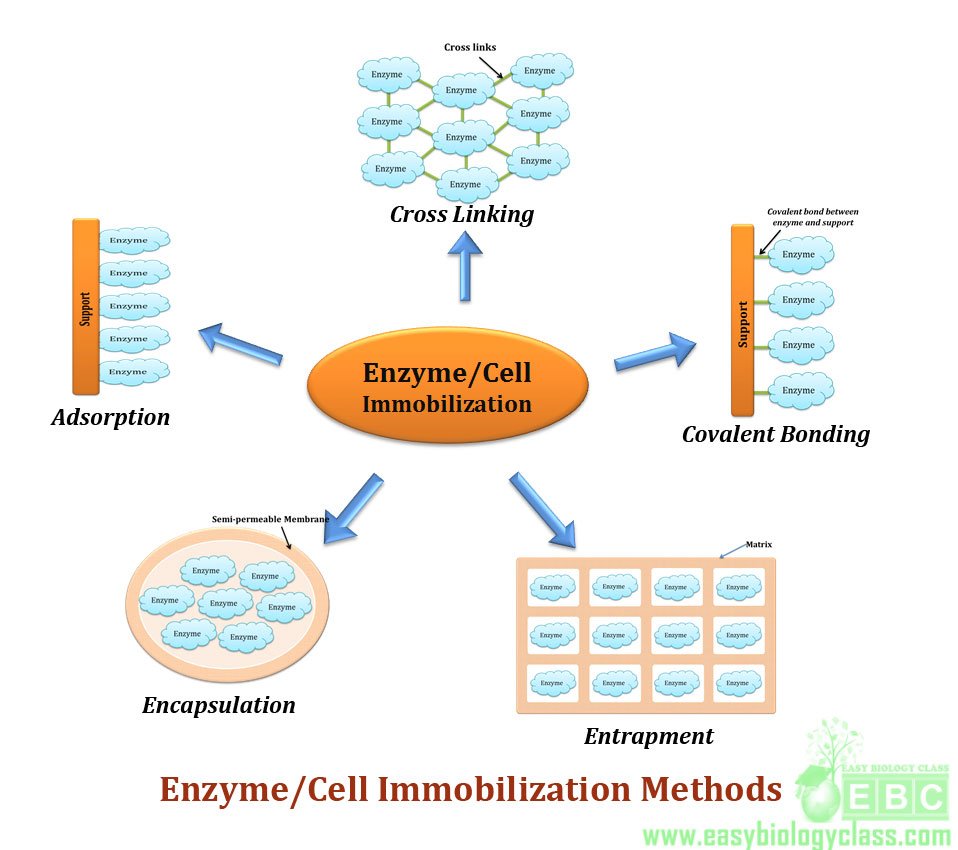
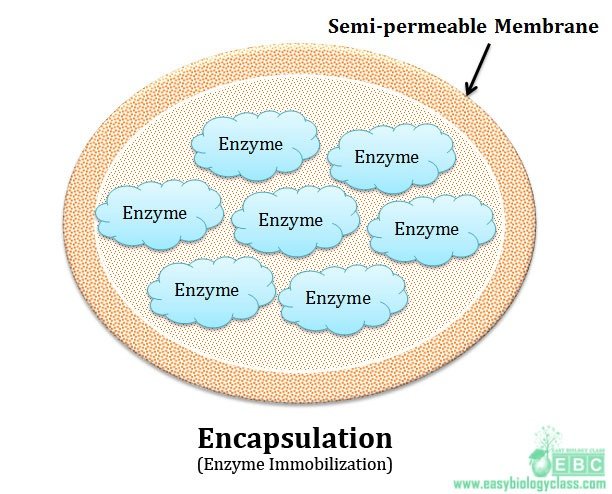
RECOMBINANT DNA TECHNOLOGY
This field of biotechnology encompases a number of
methodologies that enable new combination of genetics material to be
artificially construted in laboratory.
In other words
Recombinant DNA technology involves using enzymes and various laboratory techniques to manipulate and isolate DNA segments of interest. This method can be used to combine DNA from different species or to create genes with new functions. The resulting copies are often referred to as recombinant DNA.
rDNA
Recombinant DNA is a form of the artificial DNA that
is made through the combination or insertion of one or more DNA strands,
therefore combining DNA sequences as per your requirement.
DICOVERY
·
Discovery of DNA
structure- 1953 watson and crick.
·
Isolation of DNA
ligase- 1967 (for the formation of phosphodiester bond with the help of ATP).
·
Isolation of
restriction endonucleases around- 1970.
·
Formation of
recombinant DNA – 1972.
·
First human
protein made by using rDNA technology- Somatostatin in E.coli.
·
First plasmid
vector capable of replicate within host 1973.
RECOMBINANT DNA TECHNOLOGY GOALS
·
To isolate and
characterize a gene.
·
To make desired
and alternation in one or more isolated gene.
·
Transfer
transgene into living cells.
·
Artificially
synthesize new gene.
·
Understanding
the heriditory disease & the cure.
·
Improving human
genome.
Steps
involved in rDNA experiment-
Identification
of desired gene
Isolation of
desired gene with the help of restriction enzyme
The vector
cut with theb same restriction enzyme Vector and
isolated gene is ligated together with help of DNA Amplification
and transfer of desired gene into the progeny The
recombinant vector is introduce into the host cell
Gene cloning
The basic steps in a gene cloning experiment are follows.
1.
A
fragment of DNA containing the gene to be cloned, is inserted into a circular
DNA molecule called vector, to produce a chimera or recombinant DNA molecule.
2. The vector act as vehicle that transport the gene into a host cell, in which is usually a bacterium, although other types of living can be used.
3. Within the host cell